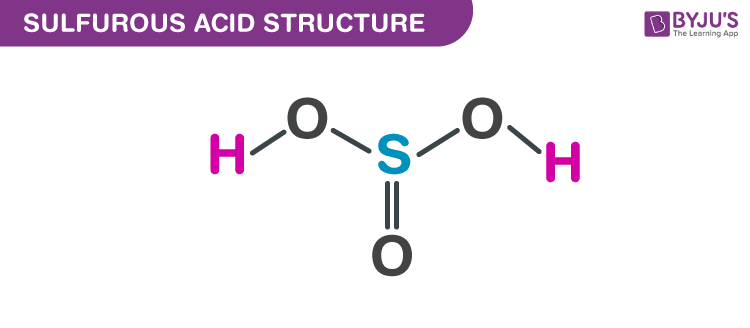
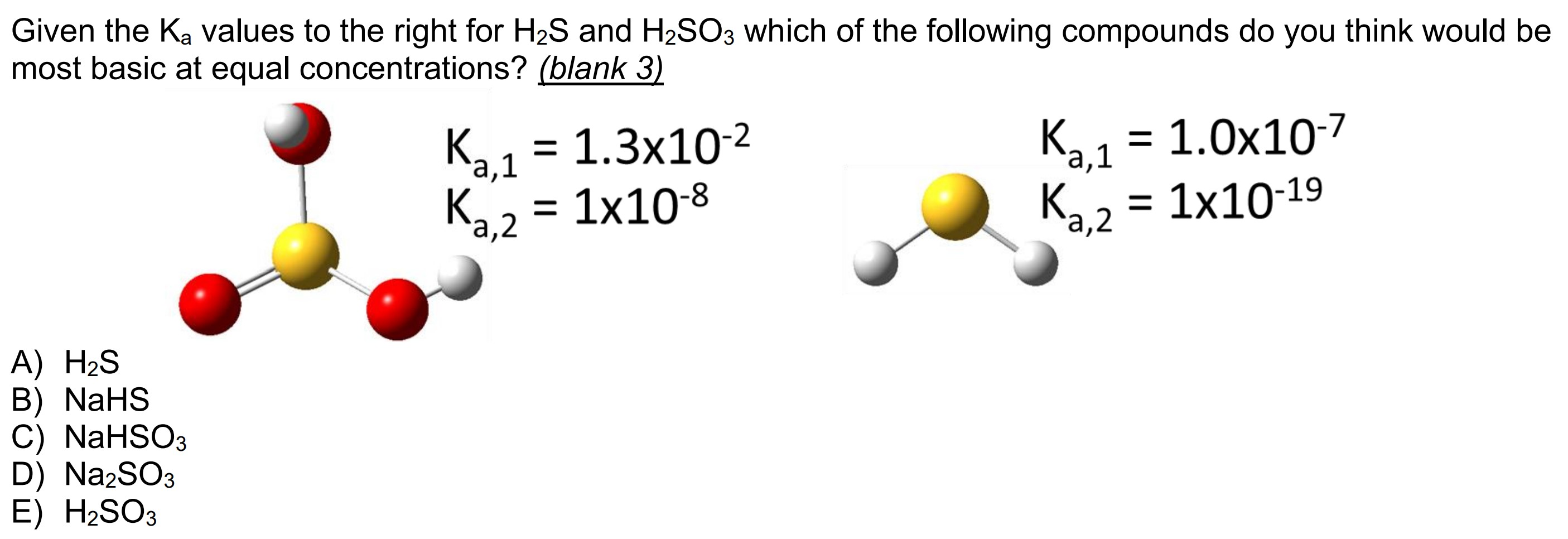
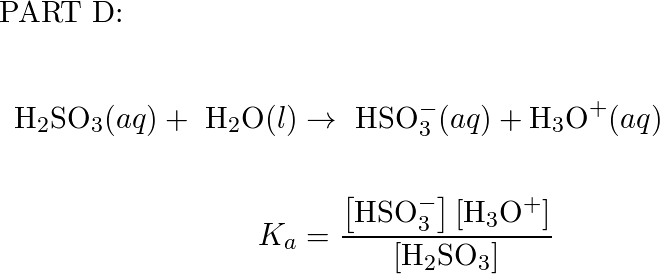Sulfurous acid, H2SO3 has acid dissociation constants Ka1 = 1.5 × 10-2 and Ka2 = 6.3 × 10-8. ... - Biology Forums Gallery
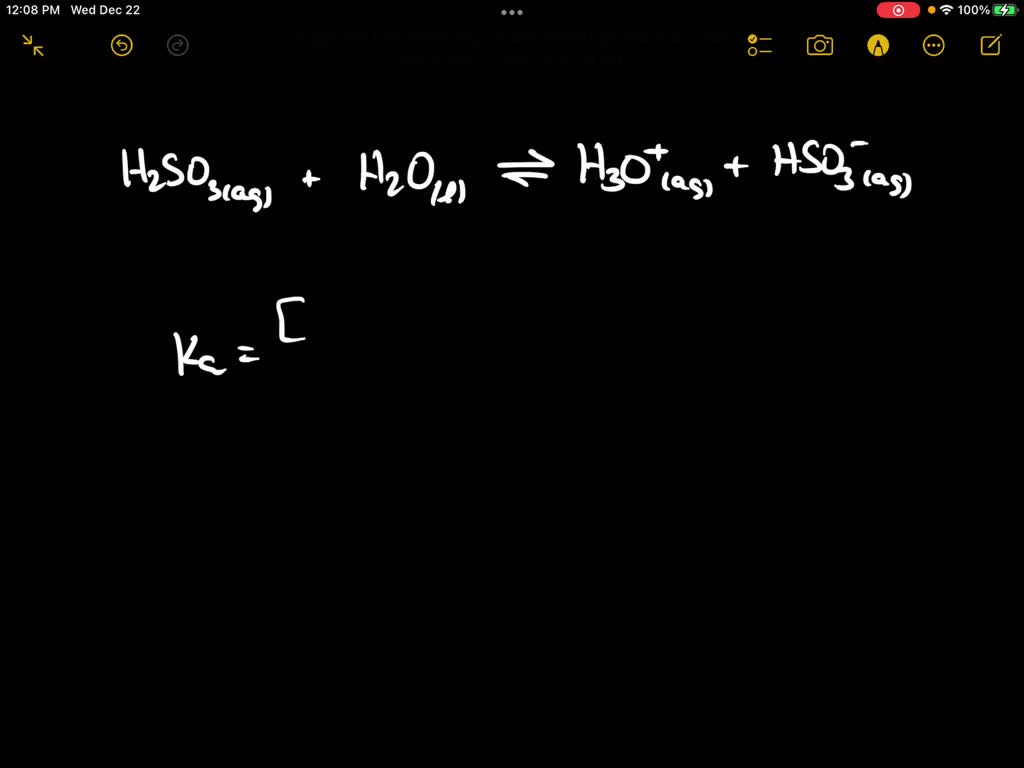
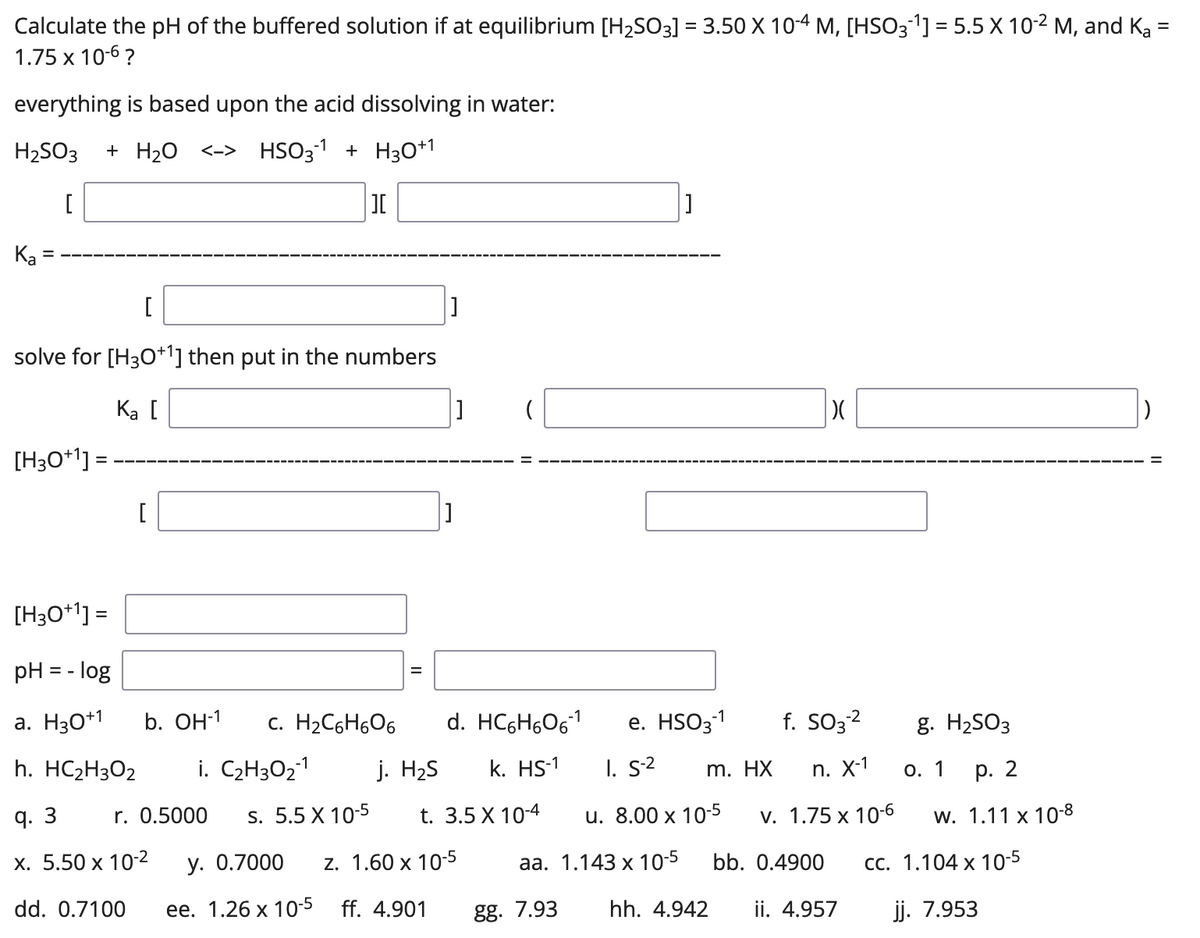
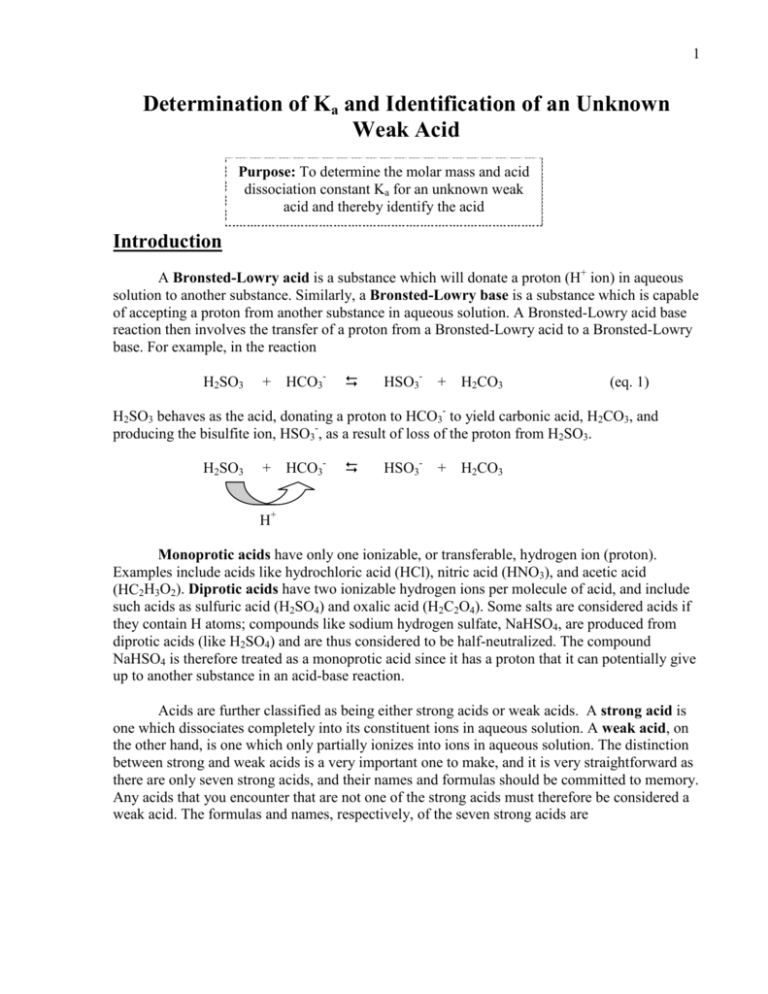
SOLVED: The equations below show the ionization reactions and the corresponding Ka values for the two ionizations of sulfurous acid, H2SO3. H2SO3(aq) + H2O(l) = H3O+(aq) + HSO3-(aq) Ka1 = 0.017 HSO3-(aq) +
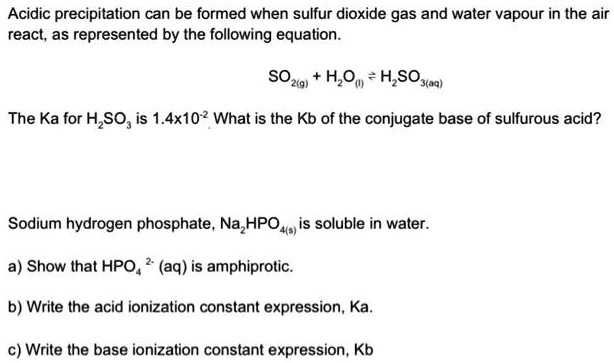
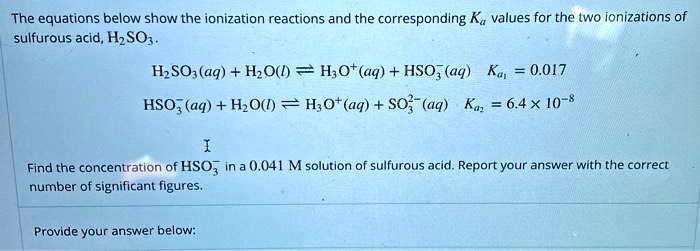
SOLVED: Acidic precipitation can be formed when sulfur dioxide gas and water vapor in the air react, as represented by the following equation: SO2(g) + H2O(l) â†' H2SO3(aq) The Ka for H2SO3
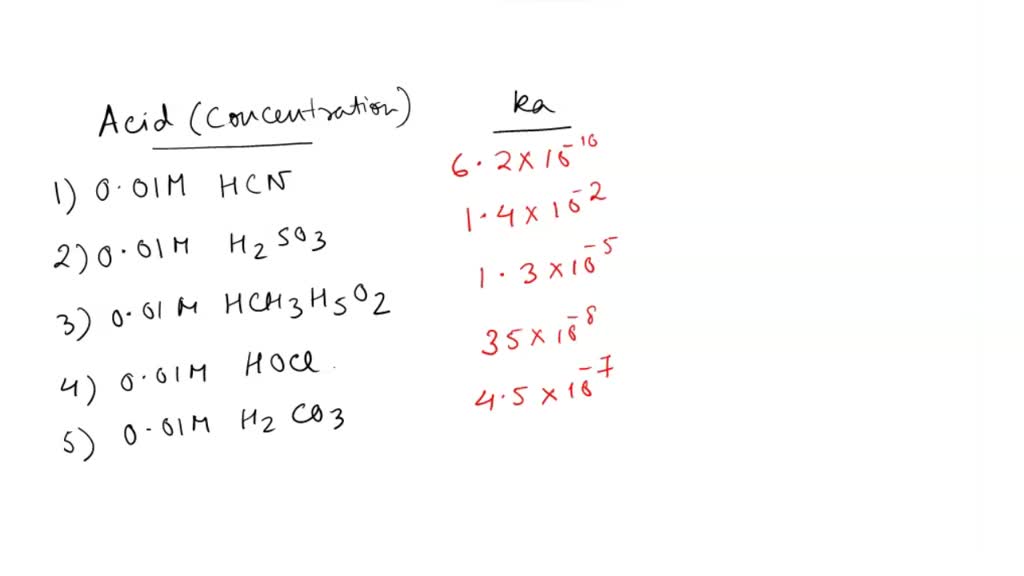
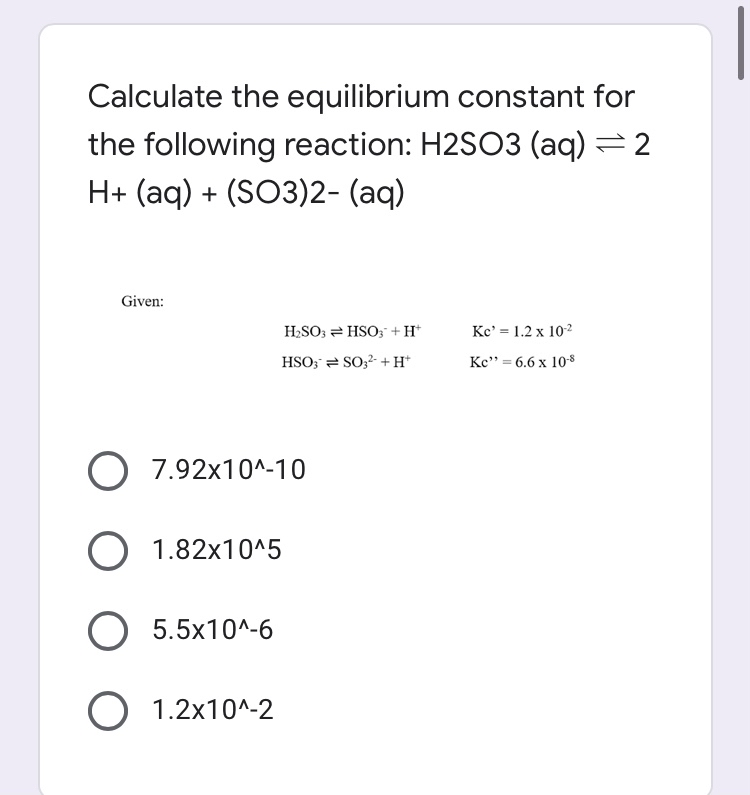
SOLVED: 'QUESTION 3 In which of the following aqueous solutions does the weak acid exhibit the highest percentage ionization? 0.01 M HCN (Ka = 6.2 x 10-10) 0.01 M H2SO3 (Ka =
Sulphurous acid (H2SO3) has Ka1 = 1.7 × 10^–2 and Ka2 = 6.4 × 10^–8. The pH of 0.588 M H2SO3 is ..... - Sarthaks eConnect | Largest Online Education Community
_how-to-balance-so2-h2o-h2so3-sulfur-dioxide-water-preview-hqdefault.jpg)
How to Balance SO2 + H2O = H2SO3 (Sulfur dioxide + Water) from sulphur dioxide formula Watch Video - HiFiMov.co